There is a lot of buzz about hydrogen as a clean fuel of the future. Looking a bit closer, the pictures is not as clear cut as many proponents would like. Fuel-cell vehicles (FCVs) are promoted by some auto makers as an alternative to battery-electric vehicles (BEVs), while BEVs and their plug-in hybrid (PHEV) cousins are starting to make significant inroads in new vehicle purchases. Yet, the case for hydrogen is far from compelling economically as well as environmentally. The main argument in favour of FCVs is the higher energy density of hydrogen compared to lithium-ion batteries. This gives hydrogen a technical advantage for long-range driving and heavy-duty vehicles. However, this advantage is offset for passenger vehicles by a decidedly less advantageous energy and greenhouse gas (GHG) footprint. Making hydrogen from natural gas—the dominant source of hydrogen today—means that FCVs are a good distance from being "zero emission vehicles" (ZEVs). Even making hydrogen from water with renewable energy requires a significant amount of extra electricity, and this results in a huge efficiency loss compared to taking electricity directly from the grid and charging batteries. Understanding hydrogen and fuel cells requires understanding where this technology can be useful, and where it can not. Let me examine some of the key issues.
How do fuel cell vehicles work?
Hydrogen fuel cells, as the image below shows, combine oxygen and hydrogen through a proton membrane to generate electricity, heat, and water. There are no harmful emissions from this process itself. Oxygen is taken from the air, while hydrogen is supplied either in liquid form on in compressed gas form. One kilogram of hydrogen at room temperature occupies eleven cubic meters. Compressed to 700 bars, hydrogen achieves a density of 42 kg/m3, and five kilograms of hydrogen (which suffice for propelling a car about 500 km) can be stored in a 125 liter (L) tank. Compressed hydrogen is the technology of choice as handling liquid hydrogen is complicated. Cooled to minus 253°C, five kilograms of hydrogen would require only a a 75L tank, but handling such liquids requires strongly insulated tanks and refueling adapters. By comparison, advances in materials science make compressed hydrogen storage the easier and cheaper option.
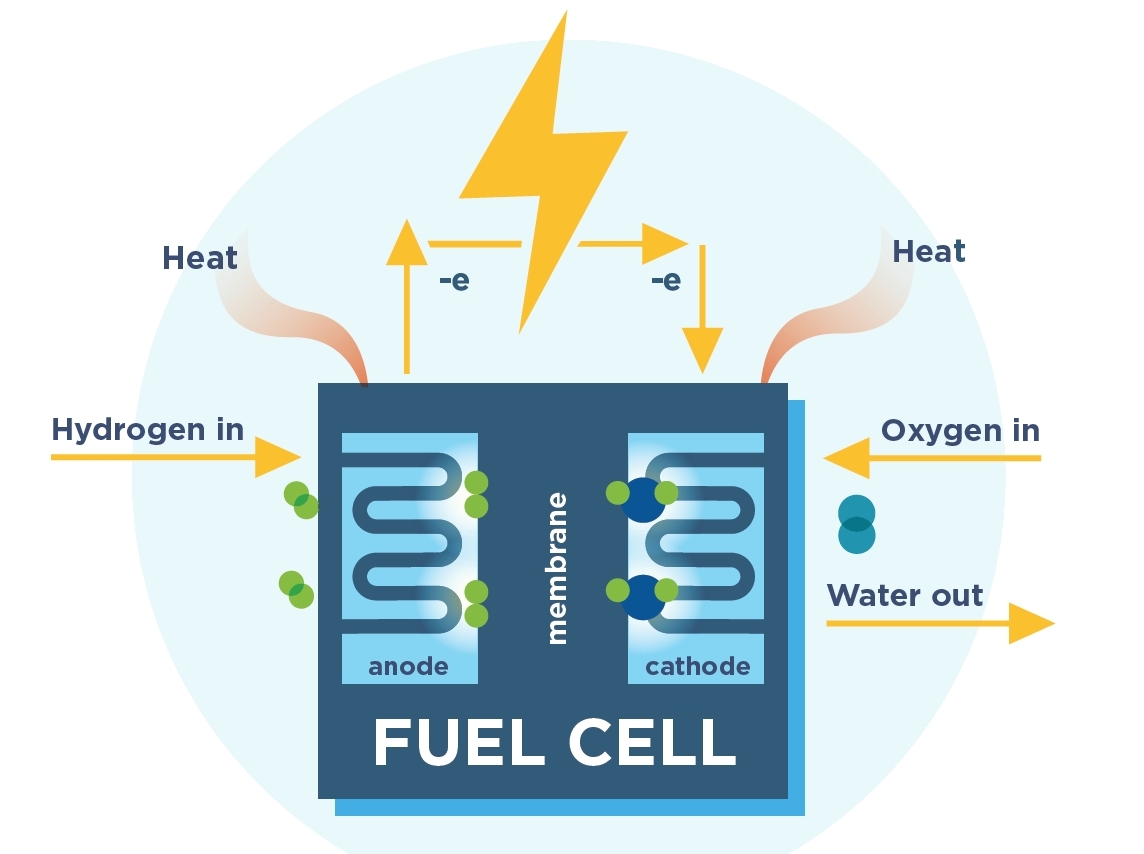
Image source: Fuel Cell and Hydrogen Energy Association
Fuel cells are not perfectly efficient converting hydrogen into electricity. Some of the energy is lost as heat in the process. The tank-to-wheel efficiency of fuel cells is reckoned to be only about 40–45 percent. Toyota reports that its FCV requires about 0.8kg of hydrogen per 100 km, although 1kg per 100km is probably more realistic under normal road conditions. A typical BEV consumes about 20-25 kWh per 100 km, depending on weight and other characteristics. Some small BEVs are even more frugal.
How is hydrogen made?
Currently, the main method of producing hydrogen is from steam methane reforming (SMR). High temperature steam (700-1,000°C) is combined with methane under 3-25 bar pressure in the presence of a catalyst to produce hydrogen and carbon dioxide in two chemical steps. Steam and methane generate carbon monoxide and hydrogen, and then a water-gas shift reaction combines carbon monoxide with more water to form carbon dioxide and hydrogen. A third step removes impurities from the gas stream, leaving pure hydrogen. Using low-cost natural gas is currently the most affordable option to make hydrogen, but it is not GHG neutral because of the carbon dioxide that is created in the process, and the heat that is needed in the process and comes from burning natural gas. Producing one kilogram of hydrogen is associated with about 9 kilograms of carbon dioxide (Sun et al., 2019).
A cleaner method of producing hydrogen is through electrolysis, where an electric current breaks water into its two constituents. If the electricity comes from clean renewable sources, the hydrogen is indeed mostly emission free. The only emissions are associated with the upstream manufacturing of power generation equipment.
Hydrogen made from SMR with carbon capture and storage is also called "blue hydrogen", while hydrogen made from water electrolysis is called "green hydrogen".
There are other pathways to produce hydrogen. One alternative technology is known as thermal and plasma pyrolysis, which breaks up methane into hydrogen and carbon directly. Plasma pyrolysis requires 10-12 kWh/kg and has far fewer emissions than SMR.
How much electricity is needed to make hydrogen?
A completely efficient electrolysis system would require 39 kWh of electricity to produce 1 kg of hydrogen. However, the devices commonly found in operation for this process are less efficient. A typical operational figure is about 48 kWh per kg of hydrogen.
How efficient are FCVs compared to BEVs?
‘BEVs are more than three times more efficient than FCVs.’
There are two types of energy losses that need to be accounted for when calculating the kWh needed to drive 100 km: well-to-tank (WTT) that accounts from source to hydrogen tank, and tank-to-wheel (TTW) in terms of fuel cell efficiency and propulsion in the vehicle. Combined, these two energy losses translated into a well-to-wheel efficiency (WTW). A recent study by Whitehead et al. (2018) shows that FCV only have a 22% efficiency, compared to 67% for BEVs. This translates into an energy use of 87 kWh per 100km for FCVs and 28 kWh per 100 km for BEVs. In other words, BEVs are more than three times more efficient than FCVs.
If FCVs are less efficient than BEVs, do they have a future?
FCVs for passenger vehicles makes neither economic nor environmental sense. Economically, the extra cost of producing hydrogen and distributing hydrogen makes FCVs less economical than BEVs. Whereas batteries are becoming continuously cheaper and significant innovation is visible on the horizon, the potential for reducing the cost of fuel cells or making them more efficient appears to be limited.
FCVs for passenger vehicles also do not make sense environmentally as long as electricity is not completely clean, or hydrogen comes primarily from SMR sources rather than renewable energy. FCVs with hydrogen from natural gas are not significantly better than modern (mild-hybrid) gasoline engine vehicles. Every extra kWh of electricity that is needed for making hydrogen is putting more carbon dioxide into the air than a battery-electric vehicle, even including for upstream emission in battery manufacturing.
‘Hydrogen FCVs are most promising for applications requiring high power density: buses, trucks, & trains.
Yet, there is some potential for FCVs—just not for passenger cars. Batteries are heavy, and where significant amounts of power are needed they are an impediment to transporting heavy loads. Because of the higher energy density of hydrogen compared to batteries, FCVs have an advantage for heavy duty vehicles: trucks and buses. Beyond motor vehicles, there is also potential use of hydrogen for rail and marine transportation, replacing diesel fuels. For these types of vehicle, the lesser efficiency of FCVs is offset by the need for power density. Still, even heavy-duty FCVs will become environmentally compelling if hydrogen is produced entirely from renewable sources. Until then, using compressed natural gas (CNG) directly in heavy-duty vehicles makes more sense economically and environmentally.
Coradia iLint, produced by French train maker Alstom, is the world's first hydrogen-powered train. It is operated in Germany's state of Lower Saxony, connecting the cities Cuxhaven, Bremerhaven, Bremervörde and Buxtehude. This technology is also called hydrail. Just recently, Alstom's hydrogen train also entereed regular passenger service in Austria.

Image source: Clean Energy Canada
What are the implications for public policy?
The above insights have very strong implications for public policy directed at decarbonizing our vehicle fleet. First, no subsidies should be provided for passenger vehicle FCVs. The future clearly belongs to BEVs in this domain. Second, as long as electrolysis from renewable energy is too expensive compared to steam-methane reforming, hydrogen is not an environmentally friendly alternative. We might as well use natural gas directly without transformation losses. If and when clean hydrogen becomes available, hydrogen may help transition to clean mobility for heavy-duty vehicles. Research and development assistance should therefore focus exclusively on applications requiring high power density.
The Government of Canada and provincial governments across Canada currently provide purchase incentives for FCVs. British Columbia and the federal government both provide $5,000 for qualifying FCVs. As long as hydrogen FCVs are not Zero-Emission Vehicles (ZEVs), they should not qualify for purchase incentives. The inclusion of FCVs in these programs is not in the public interest and does not help reduce GHG emissions. While it is true that FCVs can provide emission reductions, they are simply not as strong as from BEVs. There is also no reasonable expectation that a hydrogen distribution infrastructure will emerge. A report last year in the Globe and Mail mentioned that there are only two hydrogen refueling stations in Canada.
Car manufacturers pursuing passenger FCVs are wasting their investments. Hydrogen is simply not the appropriate solution in this category. Car buyers are already voting with their pocketbooks: BEVs are the clear winner. The future of hydrogen mobility belongs to trucks and buses, and heavy transportation vehicles of other types.
Public support for hydrogen is better directed at the applications that clearly benefit from high power density: heavy-duty vehicles and trains that replace the use of diesel fuel. Where government help is also needed is in upstream technology: producing clean hydrogen. The British Columbia Hydrogen Study explores this topic in greater detail.
How expensive is hydrogen?
A June 2019 report by the International Energy Agency "The Future of Hydrogen" reports productions costs of roughly 1 USD per kilogram of hydrogen. The cost would incrase to 1.5-2.4 USD per kilogram if carbon capture and storage is added to the prevalent SMR technology to produce hydrogen from natural gas. Less than 0.1% of global hydrogen production today comes from water electrolysis. The U.S. National Renewable Energy Laboratory (NREL) has explored the potential cost of water electrolysis in a 2011 report U.S. Geographic Analysis of the Cost of Hydrogen from Electrolysis. The numbers are not encouraging: USD 3.74–5.86/kg. The IEA looks at a possible range of USD3.00–7.50/kg in 2018. For comparison, recall that 1 kg of hydrogen gives you about 100 km of driving range. So 100km will cost about CAD 6.50, while an 8L/100km gasoline car would cost CAD 11.0 at a gasoline price of $1.375/L for driving the same 100km. So hydrogen could indeed be cheaper than gasoline. In Canada, where electricity is cheaper than in the United States, the cost would be lower. However, the main caveat remains that current hydrogen is all from natural gas (and some coal), and is far from clean. To make hydrogen clean requires adding carbon capture and storage to SMR production, or switching to (more expensive) water electrolysis from renewable sources.
What's the bottom line, then?
Hydrogen has an important role to play in our energy future—just not everywhere. For numerous applications requiring high energy density, hydrogen is just right. For others, such as passenger vehicles, hydrogen compares less favourably to batteries. Yet, many countries are investing into hydrogen. Germany is investing €9 billion into "green hydrogen" and has set a target of 5 GW of electrolysis capacity by 2030, which I calculate as equivalent to about 300,000 metric tonnes of hydrogen per year (MTA). (Note: worldwide production of hydrogen in 2018 was about 74 MTA.) All considered, hydrogen is a needed technology for a range of applications, while it is not a substitute for developing cheaper and better batteries for EVs.
Do hydrogen fuel cells have applications aside from mobility?
While hydrogen's future for transportation has limitations, hydrogen fuel cells can also be advantageous in a number of other applications. The advantage of hydrogen is its ability to be stored safely for long durations, and its ability to be transported over long distances. This enables the use of hydrogen fuel cells for backup power generation and even larger-scale storage, if it can be done economically. Whether hydrogen will emerge as the tecnological winner for energy storage depends on the alternatives, such as redox flow batteries. A crucial number to look at is the turnaround efficiency: the percentage of electricity that comes of of the storage system compared to what has gone in. Other grid-scale storage systems have higher turnaround efficiency than hydrogen. If hydrogen is unable to compete with such alternatives, its future for storage will be limited to niche applications.
Further readings and information sources:
- Jake Whitehead, Robin Smit, and Simon Washington: Where are we heading with electric vehicles?, Transport Energy, October 2018.
- Stephen Edelstein: Report: Hydrogen for fuel-cell vehicles likely to reach price parity with gasoline by 2025, Green Car Reports, June 9, 2020. See Renewable Hydrogen Transportation Fuel Production.
- Hydrogen Basics, U.S. Department of Energy, Alternative Fuels Data Center
- The Future of Hydrogen, International Energy Agency, June 2019.
- The Canadian Hydrogen and Fuel Cell Association and the U.S. Fuel Cell & Hydrogen Energy Association.
- Union of Concerned Scientists: How Clean Are Hydrogen Fuel Cell Electric Vehicles?, 2014.
- Mark Richardson: Hydrogen-powered cars are the future. But are we ready?, The Globe and Mail, 10 July 2019.
- G. Saur and C. Ainscough: U.S. Geographic Analysis of the Cost of Hydrogen from Electrolysis, U.S. National Renewable Energy Laboratory, December 2011.
- Hydrogen from Renewable Power: Technology Outlook for the Energy Transition, International Renewable Energy Agency (IRENA), September 2018.
- British Columbia Hydrogen Study, June 2019.